- RWD
Unicancer provides support to expert groups responsible for addressing questions on therapeutic strategies and research.

Launched in 2014 by Unicancer R&D and supported by all French Comprehensive Cancer Centres (FCCCs), the ESMÉ (“Épidémio-Stratégie Médico-Economique”) programme, an independent academic initiative, aims to centralise the real worls datas of patients treated for cancer in France.
The objective of ESME is to describe the evolution of patient management and therapeutic strategies over time, in a large-scale medico-economic approach.
Three data platforms have already been set up in metastatic breast cancer, ovarian cancer and lung cancer.
METASTATIC NON-SMALL CELL LUNG CANCER
+61,000 patients registered in 2024

METASTATIC BREAST CANCER
+35,000 patients registered in 2024

OVARIAN CANCER
+13,000 patients registered in 2024
For each pathology or therapeutic area, the ESME data platform relies on anonymised data documented by health professionals.
This programme therefore makes it possible to generate knowledge that is complementary to that obtained from randomised clinical trials.
Available to the scientific and medical community (researchers and clinicians), ESME enables the development of research on real-life cancer treatment in France (therapeutic strategies, determinants, efficiency, etc.). It also provides independent data to support the French health authorities in their health product evaluation missions.
Thus, the ESMÉ programme is intended to become a potential tool for decision-making in health care.
The constitution of an ESME database is currently fed from three sources of information:
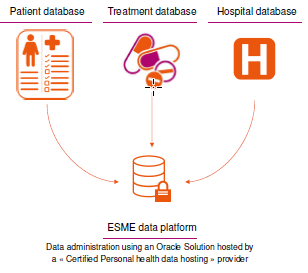
Thus, no new data is created: ESME allows existing data from the participating FCCCs and university and general hospitals to be structured in a common format for better understanding and analysis (longitudinal retrospective data).

The ESMÉ programme aims to create the largest platform of real-life data on patients treated for cancer in France. Its enrichment with data from other public and private hospitals will help achieve this goal.
Anne-Laure Martin, Directrice des données de santé en oncologie et des partenariats
The platform is supported by major industrial partners: Pfizer (whose support for metastatic breast cancer has been renewed in 2020 for 3 years), Roche, AstraZeneca, MSD, Daiichi Sankyo, Esai, BMS (as coordinator of the international partnership programme IO-Optimise for the optimisation of immunotherapy in advanced or metastatic lung cancer, in which Unicancer participates) and, since 2020, GSK (for ovarian cancer), Janssen and Amgen (the latter two for advanced or metastatic lung cancer).